A Comparison of Ion Chromatography to Membrane Diffusion/Conductivity Detection for the analysis of Agricultural Tile Samples
Tile drainage is a type of drainage system that removes excess water from the soil below the surface. The practice dates back to 200B.C. but intensive use of tile drainage began in the 1800s in Europe with the development of ceramic tile (Figure 1). In the 19th century a C-channel tile atop a flat tile were often used. (Figure 2). Today “tile lines” are typically PVC, although some are still cast concrete or ceramic. It is important to remove excess water since healthy root growth requires oxygen as well as water. If water saturates the soil in the area where roots grow, the roots become starved for oxygen. The tile drainage system moves water away from the root soil level to prevent this. Tile lines are typically placed at least 3 feet below the soil level and drained to a common drainage ditch adjacent to the planting field (figure 3).
Since the water in tile lines has carried fertilizer through the soil, tile lines also provide a fortuitous place to sample irrigation water for nutrients to ensure crops aren’t over or under fertilized. Figure 4 shows irrigation water sampling from a typical tile line. Nitrate is one of the most important nutrients to monitor.

Figure 1
Modern Day Ceramic Tile

Figure 2
Cast Cement Tile

Figure 3
Plastic Tile Line and Tile Drainage Ditch

Figure 4
Irrigation Water Sampling from a Tile Line
Recently, a Soy Bean Association sent ten blind samples to an agriculture analytical contract lab. The intent was to compare ion chromatography (IC) nitrate analysis to the Timberline Ammonia/Nitrate Analyzer nitrate analysis which employs flow injection, membrane diffusion/conductivity for the determination of ammonia. To analyze for nitrate in aqueous samples the Timberline Analyzer uses an activated zinc reduction column. The samples are passed over the zinc reduction column prior to analysis where the nitrate in the tile samples is quantitatively reduced to ammonia. The zinc reduction column is capable of reducing nitrate to ammonia for several hundred to one thousand samples depending on the concentration of nitrate present in the sample.
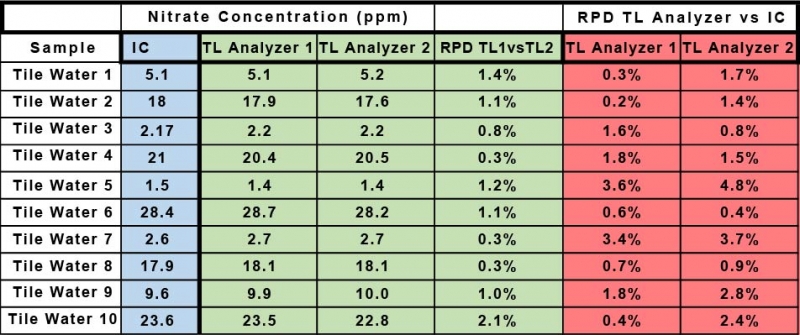
The samples were previously analyzed for nitrate concentration using ion chromatography. The contract lab analyzed them for nitrate using two different Timberline Ammonia/Nitrate analyzers. The tile samples ranged in concentration from a low of approximately 1.5 ppm nitrate-N to a high of almost 30 ppm nitrate-N. The contract lab submitted their data and the Soy Bean Association returned both the IC data and the Lab’s data for comparison. Below are the results of the comparison.
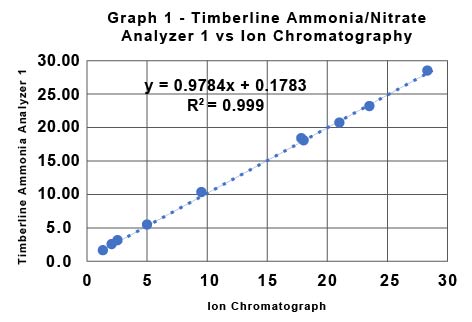
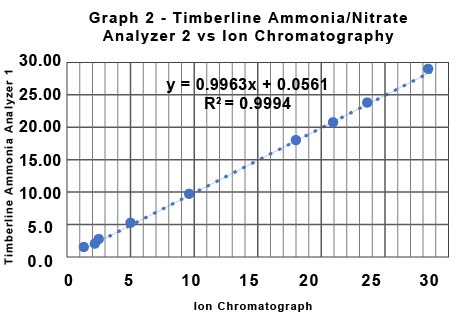
Table 1 shows the data for the determination of nitrate by IC provided by the Soy Bean Association and the data collected by the commercial lab for the determination of nitrate using the Timberline Ammonia/Nitrate analyzer (TL Analyzer). The Relative Percent Deviation (RPD) for the two sets of data for nitrate determined by the two Timberline Analyzers is in all cases less than 2%. The RPD for the Timberline Analyzer 1 vs IC ranges from a minimum of 0.3% to a maximum of 3.6%. The RPD for the Timberline Analyzer 2 vs IC ranges from a minimum of 0.8% to a maximum of 4.8%. Graph 1 and Graph 2 show the same data from a different perspective. They show the raw data in ppm for each method plotted against each other. Graph 1 is TL Analyzer 1 data vs IC data, and Graph 2 is TL Analyzer 2 data vs the same IC data. If the two techniques were exactly equal in performance the slope of the trend line would be 1. Notice that the slope for Graph 1 is 0.9978 with an R2 of 0.999, and for Graph 2 is 0.996 with an R2 of 0.9994 indicating an excellent correlation between the two techniques.
In general, the Membrane Diffusion/Conductivity Detection method for the determination of nitrate compares very favorably with Ion Chromatography for the determination of nitrate in agricultural tile samples. The Membrane Diffusion/Conductivity Detection method for the determination of nitrate in samples is simpler than Ion Chromatography, in that there are no chromatographic columns or eluents that require special care, and it is faster with a typical analysis time of about 2 minutes. It also has the advantage of being able to analyze for ammonia as well as nitrate with minor changes to the analyzer setup as compared to ion chromatography where a complete change in chromatographic setup including eluent and column is required to analyze for ammonia.